Abstract
Introduction
Presence of FLT3-ITD gene mutations is a poor prognostic factor in acute myeloid leukemia (AML). Midostaurin, a multikinase inhibitor, is approved for treatment of patients with newly diagnosed FLT3 mutant AML, in combination with cytarabine and daunorubicin standard chemotherapy. Midostaurin is not indicated as a single-agent induction therapy for AML treatment. Newer FLT3 inhibitors such as Quizartinib, Crenolanib and Gilteritinib have shown promising single agent activity in clinical trials involving patients with relapsed or refractory FLT3-mutated AML. Unfortunately, initial responses to FLT3 inhibitors are not durable, and leukemia progresses in virtually all patients. While several mechanisms of resistance to FLT3 inhibitors have been proposed, occurrence of new tyrosine kinase domain (TKD) mutations are among the most frequent and important mechanisms of resistance to current FLT3 inhibitors, making the development of novel FLT3 inhibitors imperative. F691, D835, and N676 are the most common mutations occurring in the kinase domain of the FLT3 protein; these confer resistance to currently available FLT3 inhibitors. We have synthesized a novel and selective FLT3 inhibitor, KRX-101 (a 4-substituted aminoisoquinoline), that has shown a superior anti-AML activity compared to available FLT3 inhibitors in vitro and in vivo. Importantly, KRX-101 possesses favorable pharmaceutical properties and has potent activity against the D835 and F691 mutations that arise during treatment with Quizartinib and Gilteritinib.
Methods
Using commercially available starting materials, the aminoisoquinoline compound was synthesized via a Sonogashira reaction. For enzymatic activity, KRX-101 and other FLT3 inhibitors were evaluated in protein based assays targeting FLT3-ITD including those with D835Y and F691L mutations (Reaction Biology, Malvern, PA; Eurofins DiscoveryX, Fremont, CA). In anti-proliferative assays, AML cell lines were exposed to KRX-101 and other FLT3 inhibitors for 72h in a 96-well plate. Assays were terminated with an MTT-like agent. IC50s were determined by GraphPad Prism. In pharmacokinetic assays, KRX-101 was administered to female rats (n=6) intravenously (IV, single dose, 5 mg/kg) or by oral gavage (single dose, 50 mg/kg). Blood was drawn at specific time points and plasma was isolated. The plasma concentrations of KRX-101 were determined by liquid chromatography mass spectrometry at Metabolite Profiling Facility, Bindley Bioscience Center, Purdue University. For in vivo efficacy studies, 1x106 MV4-11 cells expressing firefly luciferase were injected IV into female NSG mice. Three days later, mice were sorted into groups so that baseline luminescence (ie, disease burden) was equivalent and dosing started. Mice were imaged weekly.
Results
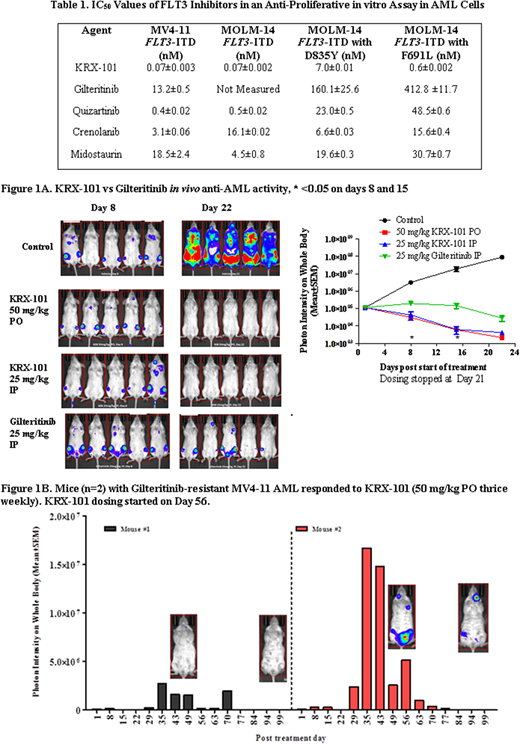
KRX-101 was synthesized with overall yield of 54% and has been scaled up for pharmaceutical development. KRX-101 in enzymatic activity assays inhibited FLT3-ITD at 2.3 nM (IC50) and FLT3-D835Y at 1.8 nM (IC50). In in vitro anti-proliferative assays, KRX-101 demonstrated robust activity in all tested AML cell lines harboring FLT3 mutations ranging from 0.07 to 7 nM (Table 1). KRX-101 showed similar or superior anti-AML activity in vitro compared to other FLT3 inhibitors. KRX-101 was readily orally bioavailable in rats. Twenty four hours after oral gavage, the plasma concentration of KRX-101 was >10 µg/mL (>18 µM); 1000 fold higher than in vitro IC50s suggesting that three times weekly dosing is reasonable. In a head to head comparative study, KRX-101 appeared to be superior to Gilteritinib in a FLT3-ITD AML orthotopic model (Fig. 1A) by inducing undetectable disease at Day 22. Additionally, two mice whose disease progressed on Gilteritinib responded to KRX-101 (Fig. 1B). In another study, animals with clearly detectable FLT3-ITD AML were treated with aminoisoquinoline for 44 days. In the absence of detectable AML at day 44, treatment was discontinued. Mice were then monitored until day 175; 4 of 5 mice (80%) had no measurable disease. In all studies, KRX-101 dosed daily or thrice weekly was tolerated well.
Conclusion
KRX-101 is a novel agent with promising activity in FLT3 inhibitor-resistant AML. Testing in other FLT3 inhibitor-resistant animal models with various tyrosine kinase domain mutations is ongoing. Investigational New Drug (IND) enabling studies are underway. The Phase I clinical trial is planned.
.
Sintim:KinaRx, LLC: Other: Founder and Scientific Advisor. Aman:KinaRx, LLC: Other: Founder. Holtsberg:KinaRx, LLC: Other: Founder. Emadi:NewLink Genetics: Research Funding. Lapidus:KinaRx, LLC: Other: Founder and Scientific Advisor.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal